Indoor Air Quality (IAQ) especially in health facilities is very important to note, not only for comfort but also for the health of patients and health workers on duty. Poor indoor air quality can have an impact on health.
Indoor Air Quality (IAQ) refers to the quality of air in and around buildings, especially regarding the health and comfort of building occupants. Understanding and controlling indoor pollutants can help reduce the risk of indoor health problems.
Poor indoor air quality can have an impact on health. Some health effects may appear immediately after single or repeated exposure to a pollutant. Eye, nose and throat irritation, headaches, dizziness, and fatigue are some examples. These effects are usually short-term and treatable, but they can sometimes worsen the symptoms of some diseases such as asthma.
Other health effects may appear years after the exposure occurred or only after prolonged or repeated periods of exposure. These effects include several respiratory diseases, heart disease and cancer.
Indoor air quality issues are considered as one of the most important topics to be assessed and monitored especially in health facilities. As a place to get treatment, treatment and recovery of disease, health facilities must have good indoor air quality.
Air quality in Indonesian health facilities is regulated in several Health Ministerial Regulations. In the Regulation of the Minister of Health of the Republic of Indonesia Number 7 of 2019, it is stated that indoor air quality conditions in health facilities have the potential to cause disease transmission.
In order to achieve compliance with the quality standards and requirements for air hygiene in the implementation of hospital environmental health, the following efforts must be carried out:
- Room air quality must always be maintained so that it does not smell, does not contain dust and gases, including asbestos dust which exceeds the provisions.
- All rooms in a hospital are designed to comply with room ventilation requirements, especially certain rooms such as operating rooms, intensive care rooms, isolation rooms, baby care, laboratories, B3 storage rooms, and other rooms that require special requirements.
- Measurement of room temperature, humidity, flow, and air pressure using appropriate environmental health measurement equipment
- Rooms that do not use air conditioning, then the arrangement of fresh air circulation in the room must be adequate with reference to statutory provisions. If you use an air conditioner, you must pay attention to the cooling tower so that it does not become a breeding ground for legionella bacteria and for AHU (Air Handling Units) the air filter must be cleaned from dust and bacteria or fungi.
- The air supply and exhaust should be mechanically actuated, and the exhaust fan should be located at the end of the ventilation system
- Room with a volume of 100 m3 at least 1 fan with a diameter of 50 cm with an air flow rate of 0.5 m3/second, and the frequency of air changes per hour is 2-12 times
- Intake of air supply from outside, except for individual room units, should be placed as far as possible, at least 7.5 meters from the exhauster or combustion equipment
- Minimum intake height of 0.9 meters from the roof
- The system should be pressure balanced
- Air supply for sensitive areas: operating rooms, baby care, taken near the ceiling and exhaust near the floor, 2 (two) exhaust fans should be provided and placed at least 7.50 cm from the floor
- Air supply above the floor
- Corridor air supply or exhaust fan exhaust from each room should not be used as air supply except for air supply to toilets, toilets, warehouses
- Ventilation of sensitive rooms should be equipped with 2 bed filters. Filter I is installed in the air receiving section from the outside with an efficiency of 30% and filter II (bacterial filter) is installed with 90%. To study the central ventilation system in buildings, you should study the central air conditioning system specifically
- Natural ventilation, ventilation holes are attempted by a cross ventilation system and are maintained so that the air flow is not obstructed
- Air conditioning in the operating room must be maintained so that the pressure is higher than other rooms and using mechanical means (air conditioner).
- Mechanical ventilation using an exhaust fan or air conditioner is installed at a minimum height of 2 meters above the floor or a minimum of 0.2 meters from the ceiling
- Air microbiology measurements can be carried out independently using appropriate laboratory equipment and measuring equipment, or can be carried out by outside laboratories that have been nationally accredited. The following is the specified Maximum Concentration of Microorganisms Per m3 of Air:
- Empty operating room = 35 cfu/m3
- Operating room with activity = 180 cfu/m3
- Ultraclean operating room = 10 cfu/m3
- To reduce the level of germs in the air, the room must be disinfected using the appropriate materials and methods
- Monitor indoor air quality at least once a year and if the use of disinfectants changes, samples are taken and air quality parameters checked
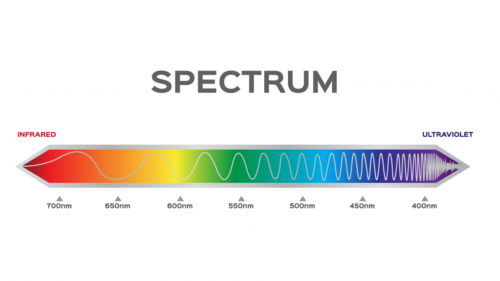
One of the ways to reduce the level of microorganisms in the air in a closed space is to use OXIRA XG (Germicidal). With Ultraviolet Germicidal Irradiation (UVGI) technology and a wavelength of 254 nm, OXIRA XG (Germicidal) will work to damage the DNA/RNA of viruses, bacteria and infectious fungi circulating in closed air, thereby limiting their ability to grow and reproduce.

Picture 1. OXIRA XG (Germicidal)
According to the journal Inactivation of Virus-Containing Aerosols by Ultraviolet Germicidal Irradiation, UVGI (Ultraviolet Germicidal Irradiation) has been scientifically proven to be effective & efficient for inactivating viruses in the air. Microorganisms in the air are more susceptible to damage from UV exposure.
In addition, OXIRA XG (Germicidal) has also passed various clinical trials in Australia, India, and Indonesia. OXIRA is proven to significantly reduce 83% the number of colonies of infectious microorganisms in the air.

Picture 2. Results of clinical trials in Australia

Picture 3. Results of Clinical Trials in India
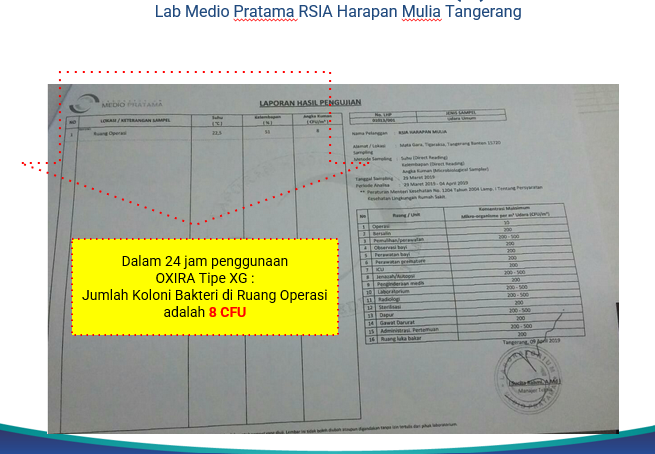
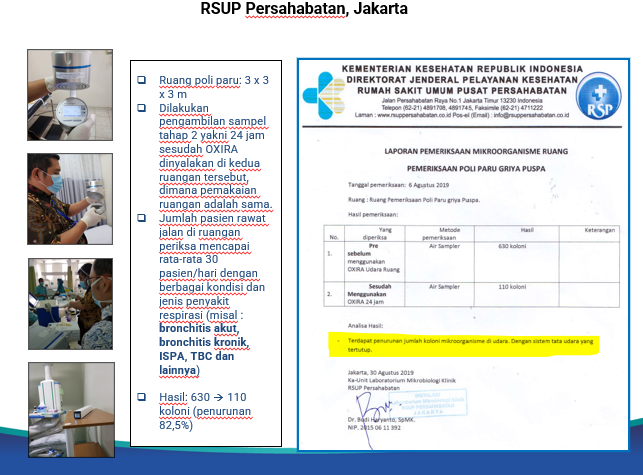
Picture 4. Results of clinical trials in Indonesia
Other information about OXIRA XG (Germicidal) can be read here and please contact us if you are interested in this product.
References:
- KEMENKES RI. (2019). Peraturan Menteri Kesehatan Republik Indonesia Nomor 7 Tahun 2019 Tentang Kesehatan Lingkungan Rumah Sakit
- Marco Gola, Gaetano Settimo, and Stefano Capolongo. (2019). Indoor Air Quality in Inpatient Environments: A Systematic Review on Factors that Influence Chemical Pollution in Inpatient Wards
- US EPA. (2022). Introduction to Indoor Air Quality