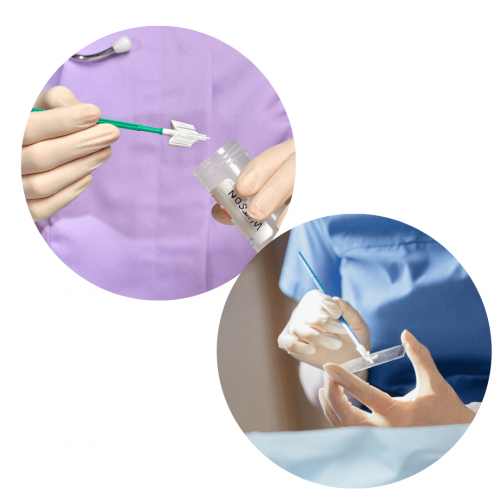
Liquid-Based cytology is a method for screening pre-cancer cytology. The development of research results and the application of technology has succeeded in developing improvements to the preparation of Pap tests, namely the LBC (Liquid Based Cytology) Pap Test method. The advantages of cytological preparation of the Pap test LBC method will be explained in this article.
Liquid-Based cytology is a method for screening pre-cancer cytology. The development of research results and the application of technology has succeeded in developing improvements to the preparation of Pap tests, namely the LBC (Liquid Based Cytology) Pap Test method. The advantages of cytological preparation of Pap test LBC method:
1. Almost 100% of the test samples can be checked
2. Standard preparation procedure, easy, fast and the results are uniform
3. Preparation results: cells are visible & a thin layer increases the accuracy in abnormal cell detection
4. Disturbances from the presence of mucus, blood, and fecal debris are removed
5. Samples are available for confirmatory tests (HPV DNA Test, Chlamydia test/ Gonorrhea)
Liquid-Based Cytology (LBC) is an accurate method of cervical cancer screening. If on a pap smear a lot of sample cells are wasted, then the LBC method is very little or even eliminated so that a more accurate diagnosis can be made, LBC has a sensitivity of 99%-100%. The process of taking a sample of cells is the same as in a Pap smear, the vagina is inserted into a funnel and the doctor takes a sample of cells from the cervix with a scraper or a small brush. The difference is if on a Pap smear the cells will be smeared onto a glass slide, in LBC the cells will be put into a bottle filled with a special liquid.
PT Isotekindo Intertama is a distributor of the CY-PREP ™ CY-100 Processor, a cervical pre-cancer cytology product using the accurate LBC method. CY-PREP ™ CY-100 Processor products are as follows:
A. Sampling using CY-PREP ™ Pap Test CERVIX-BRUM ®
Benefit:
- The sampling device is sterile, specially packaged it safe for the patient, and designed to take samples of both endo and ectocervical at the same time
- Designed to simplify sampling and reduce sampling inconvenience
B. Liquid media to contain, transport, and preserve test samples using CY-PREP™ Pap Test Preservation Solution
Benefit:
- Protect the sample, hold for 3 weeks at room temperature (15-30 °C). Samples are safe from dryness & damage
- Minimizes the risk of important samples being left in the sampling device
- Collect all cervical samples so that all sample cells can be examined
- Provide enough test samples so that the patient does not need to re-samples for follow-up tests
C. Separating the inspection target material from lenders and dirt using a dual filter
Benefit:
- Make a thin layer of preparation
- Cells become clearer when examined because the cell staining process becomes more perfect and adheres better
D. Glass slide for sample attachment using IHC link glass slide
Benefit:
- Make a thin layer of preparation
- Cells become clearer when examined because the cell staining process becomes more perfect and adheres better
When should women do a Pap test?
1. Women aged < 21 years: Pap smear screening is carried out 3 (three) years after having first sexual intercourse. If the results are normal, then it is done once a year.
2. Women aged between 21-30 years: Pap smear screening is done once a year or according to the doctor's advice if there are abnormal results.
3. Women aged > 30 years: screening with regular pap smear and HPV-DNA examinations. Women aged > 30 years who have been active have a high risk of persistent HPV infection and this is closely related to the incidence of cervical cancer.
References:
- Johnson, T. WebMD (2020). Cervical Cancer.
- Insert Pack CY-PREP ™ CY-100 Processor.
- Mayo Clinic (2021). Diseases & Conditions. Cervical Cancer.
- National Health Service (2018). Health A to Z. Cervical Cancer.
- World Health Organization (2017). Cervical Cancer Screening and Management of Cervical Pre-Cancers.